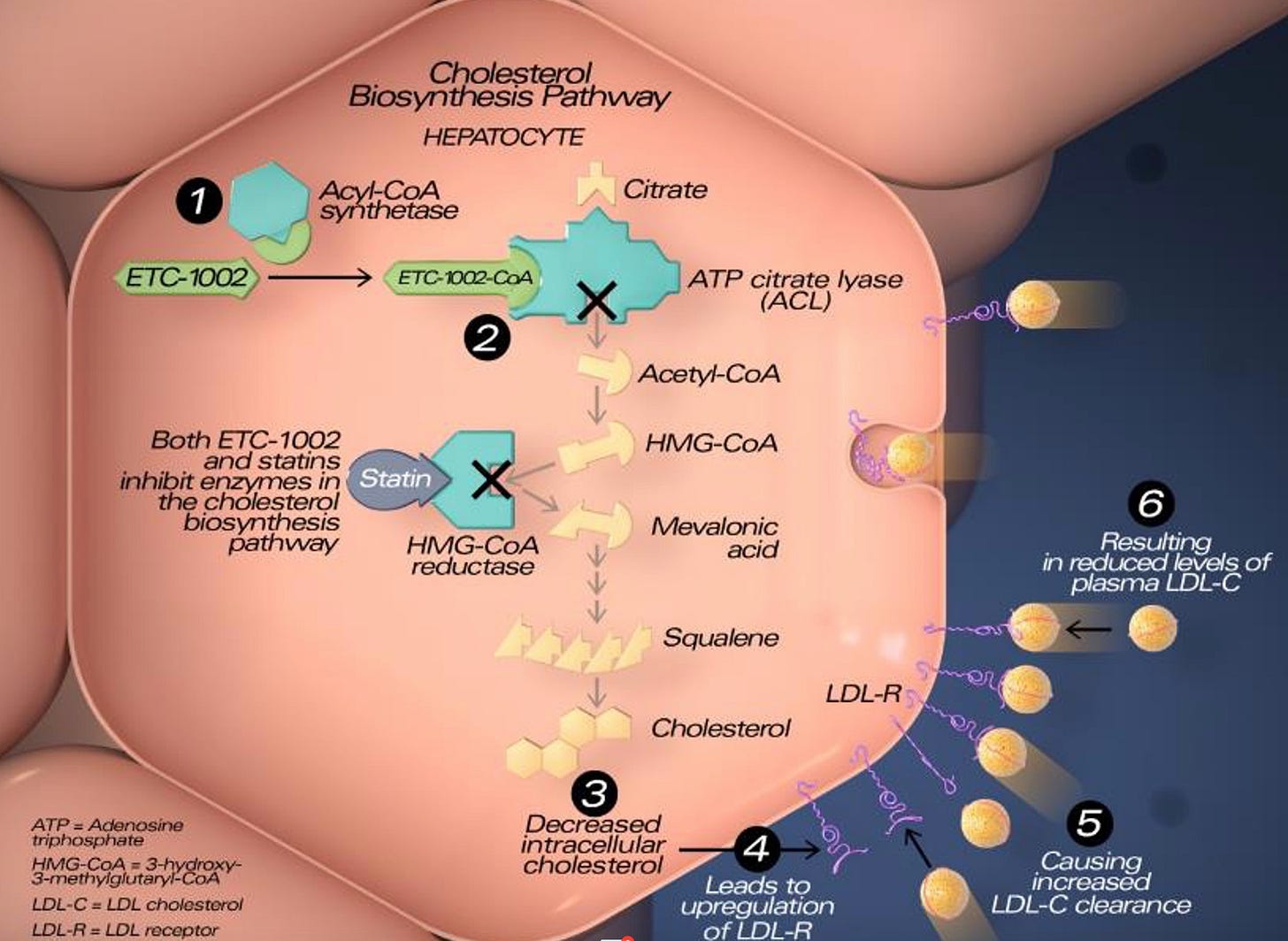
Bempedoic acid, marketed as Nexletol and when combined with ezetimibe as Nexlizet, is a citrate lyase inhibitor that reduces LDL-cholesterol (C). Citrate lyase is a not-rate-limiting enzyme in the mevalonate/cholesterol production pathway. Citrate lyase is located proximal to the rate-limiting enzyme hydroxy-methyl-glutaryl co-enzyme A (HMG Co-A) reductase. Statins inhibit HMG CoA reductase, reducing hepatic cholesterol content. The hepatocytes respond to the reduced intrahepatic cholesterol by increasing LDL receptors. These receptors take cholesterol out of the blood stream thereby reducing LDL-C. Bempedoic acid also increases LDL receptors. This is important because it appears that any cholesterol agents that reduce LDL-C by increasing LDL receptors reduce cardiovascular events.1
Before discussing bempedoic acid, I should reveal my conflicts of interest regarding the drug. I wrote the first clinical report on bempedoic acid in 2015, when it was known at ETC-1002.2 I was also on the Executive Committee for the CLEAR-Outcomes study, which was an industry-sponsored study.3 I am also a friend of Roger Newton, PhD. Roger is the founder of Esperion, which makes and markets bempedoic acid. Roger was Director of Drug Development at Parke-Davis, when atorvastatin was developed, and before Parke-Davis was purchased by Pfizer. At one point the Parke-Davis Board of Directors was not going to continue the development of atorvastatin. The Board thought atorvastatin was just another statin because Merck was already selling lovastatin and simvastatin and Bristol-Myers was selling pravastatin.. Roger literally got down on his knees before the Board to beg the Board to develop the drug. I have confirmed this with Roger. Roger worked for Pfizer after Pfizer purchased Parke-Davis, and he knew Pfizer’s pipeline. When Pfizer decided to exit the cardiovascular drug market, Roger was aware of ETC-1002, and in 2008 purchased the rights to the drug from Pfizer to develop it at Esperion.
Esperion developed bempedoic acid specifically for the treatment of statin-intolerant patients, one of my research interests. Bempedoic acid is administered orally as a pro-drug. It has to be acetylated by the enzyme, very long - chain acyl-CoA synthetase-1. This enzyme is present in the liver and at low concentrations in the kidney, but is essentially absent from skeletal muscle.4 Consequently, if bempedoic acid escapes first-pass hepatic metabolism, and gets into skeletal muscle, it cannot be converted to the active metabolite and therefore should not cause myalgia. It’s important to know this mechanism and rationale for its use. I explain this to statin-intolerant patients so they know why I think it will work in them without side effects.
The package insert states that bempedoic acid reduces LDL-C about 18%, but my study found that 180 mg of bempedoic acid, the standard dose, reduced LDL-C 28% more than placebo.2 The difference is probably because no patients in my report were on statins, whereas the studies used to inform the package insert often included some patients on some statin. A recent summary suggests that bempedoic acid alone reduces LDL-C about 24% and in combination with ezetimibe about 40%.6. Its half-life is 15 to 24 hours so it can be used off-label every other day or thrice weekly. I do this occasionally with those statin intolerant patients who are very wary of any drug.
Bempedoic acid’s effect on morbidity and mortality was studied in the CLEAR Outcomes study.3 That study randomly assigned 6,992 subjects to bempedoic acid and 6,979 to placebo. The study had a four-week, single-blinded placebo run-in where the investigators, but not the subjects, knew that everyone was taking the placebo. Only subjects who were unwilling or unable to take full-dose statin were recruited. All had a prior atherosclerotic event or were at high risk for such an event. LDL-C decreased 26.1% in the bempedoic acid subjects and 10.6% in placebo subjects. The decrease in the placebo subjects was probably because they were more likely to be started on other drugs such as PCSK9 inhibitors, which became more widely used during the study. The composite endpoint of cardiovascular death, stroke, non-fatal myocardial infarction, and coronary revascularization, decreased 13% more with bempedoic acid treatment (p=.004) than with placebo. Myalgia was slightly, but not significantly, lower in the bempedoic acid group (5.6 vs 6.8%).
Bempedoic acid is not widely used for unclear reasons. It was launched during the pandemic, which restricted marketing activities so a lot of providers do not know of the drug. Some insurance companies have not provided coverage, which is strange, since these same companies will often pay for PCSK9 inhibitors.
Clinicians should be aware of two laboratory changes with bempedoic acid. It increases uric acid levels, so it can produce gout in susceptible subjects. I do not use the drug in patients with a history of gout. It also causes a small reversible increase in creatinine. This was called “kidney injury” in the NEJM publication,3 a term I argued against as a co-author, because it really is simply an increase in creatinine because of decreased renal tubular excretion of creatinine. This is similar to the increase in serum creatinine produced by fenofibrate, which also reduces creatinine excretion. The creatinine returns to baseline with cessation of both bempedoic acid and fenofibrate, but it’s important to know that this can occur.
So, these are the rules you need to know about bempedoic acid:
- It reduces LDL-C 18 to 28% depending on whether or not the patient is also on some statin.
- Combined with ezetimibe, bempedoic acid reduces LDL-C by about 40%.
- It is administered as a pro-drug and requires acetylation to become active.
- It should not cause myalgia because the enzyme required to activate it is not present in skeletal muscle.
- This probable low risk of myalgia and why is useful information to tell patients with statin myalgia because they are often wary of new drugs.
- It has a long half-life so produces some effect with less-than-daily use.
- It increases LDL receptors and has been documented to reduce atherosclerotic cardiovascular events.
- It produces small reversible increases in blood uric acid and creatinine concentrations because it interferes with their renal excretion.
1. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA. 2016 Sep 27;316(12):1289-97. PMID: 27673306 .
2. Thompson PD, Rubino J, Janik MJ, MacDougall DE, McBride SJ, Margulies JR, Newton RS. Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol. 2015 May-Jun;9(3):295-304. PMID: 26073387
3. Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, Thompson PD, Libby P, Cho L, Plutzky J, Bays HE, Moriarty PM, Menon V, Grobbee DE, Louie MJ, Chen CF, Li N, Bloedon L, Robinson P, Horner M, Sasiela WJ, McCluskey J, Davey D, Fajardo-Campos P, Petrovic P, Fedacko J, Zmuda W, Lukyanov Y, Nicholls SJ; Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. CLEAR Outcomes Investigators.N Engl J Med. 2023 Apr 13;388(15):1353-1364. 4 PMID: 36876740
5. Pinkosky SL, Newton RS, Day EA, Ford RJ, Lhotak S, Austin RC, Birch CM, Smith BK, Filippov S, Groot PHE, Steinberg GR, Lalwani ND.Nat Commun. 2016 Nov 28;7:13457. PMID: 27892461
6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9474414/#:~:text=Bempedoic%20acid%20with%20a%20single,moderately%20improves%20the%20glycaemic%20profile.




Thank you for the kind comment.
what a great review thank you-